
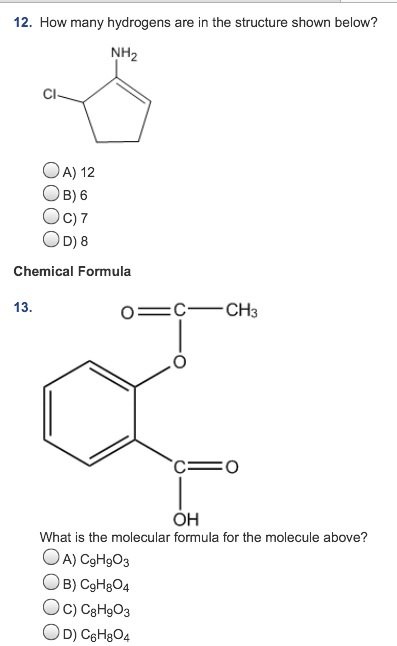
When other groups are present on the ring, it is numbered clockwise or counterclockwise depending on which direction gives the next substituent the lower number: This rule always puts the SH group at C1, therefore, the “1” is usually omitted from the name: When naming a cyclic thiol, start numbering the ring beginning with the carbon connected to the SH group. Also, if there is a double, the E and Z configuration should be addressed when applicable: If the SH group is connected to a chiral center, you will also need to include the absolute configuration at the beginning of the name. Therefore, you need to number the parent chain such that the SH gets the lowest possible number: The SH group has a higher priority than alkyl substituents or π bonds. If it is a branched molecule, choose the parent chain such that it is the longest carbon chain containing the carbon atom connected to the SH group:

The presence of the SH group is identified by changing the parent suffix from “ e” to “ thiol”:įor simple thiols, the common name can be derived by naming the alkyl group connected to the SH and adding the word mercaptan: Step 4. Put everything together having the substituents in alphabetical order.
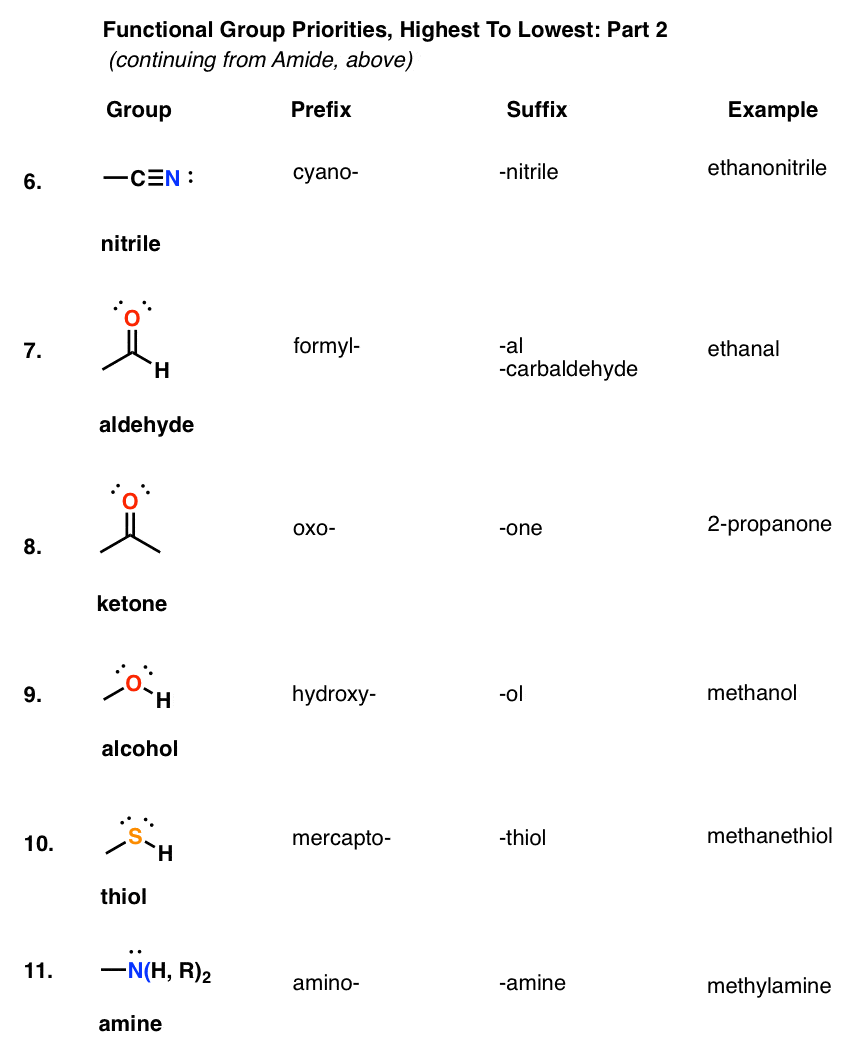
Step 3. Number the parent chain giving the carbon bonded to the SH group the lowest locant This would be a brief summary for naming a thiol: When naming a thiol, we follow the same rules we discussed earlier for the IUPAC nomenclature rules for alcohols with the focus on giving the carbon bonded to the SH group the lowest locant. Iodoacetamides and maleimides are by far the most popular thiol-reactive moieties.Thiols are sulfur analogues of alcohols, containing an SH group in place of the OH group. There are quite a few types of thiol-reactive dyes reported in the literature, including iodoacetamides, disulfides, maleimides, vinyl sulfones and various electron-deficient aryl halides and sulfonates. Thiol-reactive dyes have been used to develop probes for analyzing the topography of proteins in biological membranes, determining distances within the protein or between the proteins and monitoring the changes in protein conformation using environment-sensitive probes. Therefore thiol-reactive dyes are often used to prepare fluorescent peptides, proteins and oligonucleotides for probing biological structures, functions and interactions. Three major classes of amine-reactive fluorescent reagents are currently used to label biopolymers: succinimidyl esters (SE), isothiocyanates, and sulfonyl chlorides.īecause free thiol (SH) groups, also called mercapto groups, are not present as abundantly as amino groups in most biopolymers such as proteins and nucleic acids, thiol-reactive reagents often provide a means of selectively modifying a protein at a defined site. A number of fluorescent amino-reactive dyes have been developed to label various biomolecules, and the resultant conjugates are widely used in biological applications. In these applications, the stability of the chemical bond between the amine-reactive dye and biomolecule is particularly important because the fluorescent conjugates are often subjected to rigorous incubation, hybridization and washing steps. Amine-reactive dyes are most often used to prepare bioconjugates for immunochemistry, fluorescence in situ hybridization (FISH), cell tracing, receptor labeling and fluorescent analog cytochemistry. For fluorescent labeling dyes, most of fluorescent labeling dyes either target an amino group or thiol group.Īmine-reactive fluorescent probes are widely used to modify peptides, proteins, oligonucleotides, nucleic acids, ligands and other biomolecules. They are generally consistent of two moieties, i.e., the fluorophore as Tag, and the functional group either for conjugations or binding to a biological target. Fluorescent dyes are widely used for biological detection.


 0 kommentar(er)
0 kommentar(er)
