
Ionic properties can be exploited by chemists for a range of purposes.
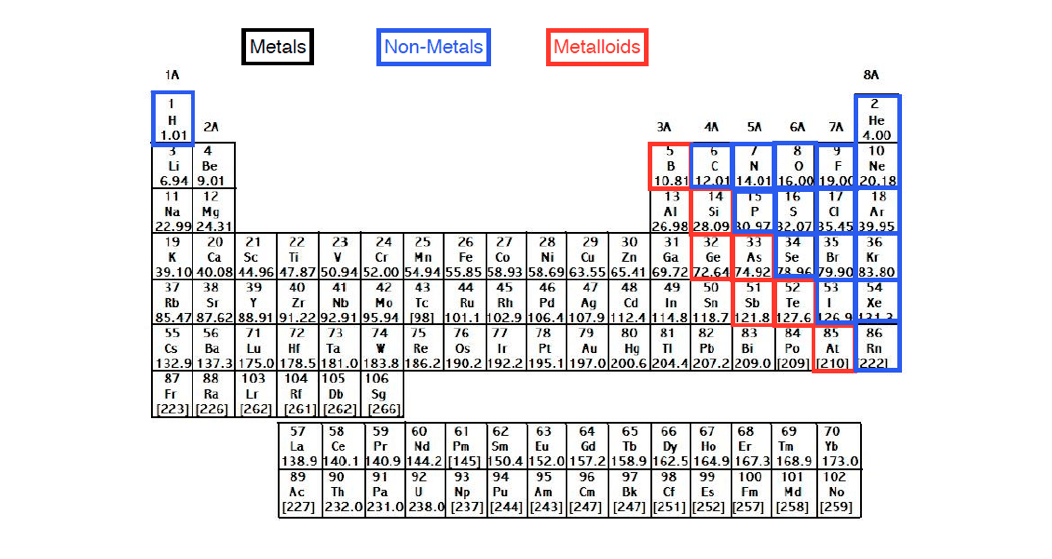
One example is hydrogen, which may gain (H -) or lose (H +) an electron, forming hydride compounds such as ZnH 2 (where it is an anion) and hydron compounds such as H 2O (where it is a cation).Įlements in group 18 of the periodic table – the “noble gases”, tend not to form ions due to the arrangement of their electrons which makes them generally unreactive. However, some elements are capable of forming both cations and anions given the right conditions. iron, silver, nickel), whilst most other nonmetals typically form anions (e.g. Halogens always form anions, alkali metals and alkaline earth metals always form cations. It can be possible to predict whether an atom will form a cation or an anion based on its position on the periodic table.

Examples include calcium chloride (CaCl 2), potassium iodide (KI) and magnesium oxide (MgO). These oppositely charged ions then attract one other to form ionic bonds and produce ionic compounds with no overall net charge. Therefore, when atoms from a metallic and a nonmetallic element combine, the nonmetallic atoms tend to draw one or more electrons away from the metallic atoms to form ions. Conversely, most nonmetallic atoms attract electrons more strongly than metallic atoms, and so gain electrons to form anions. Consequently, they tend to lose electrons and form cations. Metallic atoms hold some of their electrons relatively loosely.

Sodium (Na +), Iron (Fe 2+), Ammonium (NH 4 +)Ĭhloride (Cl -), Bromide (Br -), Sulfate (SO 4 2-) The main differences between cations and anions are summarized in the table below.


 0 kommentar(er)
0 kommentar(er)
